Merck Animal Health, a division of Merck & Co. Inc., Rahway, N.J., on Sept. 1 voluntarily recalled three batches of BANAMINE®/BANAMINE®-S (flunixin meglumine injection) 50 mg/mL in the United States, used for injection in cattle, swine and horses, due to the presence of particulate matter.
BANAMINE® /BANAMINE®-S (flunixin meglumine injection) is a prescription product in the U.S.
Affected batches
Particulates were observed during routine quality testing and reviews for the following batches:
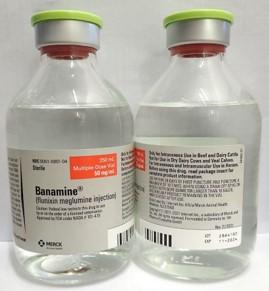
- BANAMINE 100mL, UIN 065474, NDC 00061-0851-03, Batch 2834102, exp Oct. 2024
o Distribution dates: March 6, 2023, to May 3, 2023
- BANAMINE 250mL, UIN 065476, NDC 00061-0851-04, Batch 2864102, exp Nov. 2024
o Distribution dates: June 21, 2023, to July 11, 2023
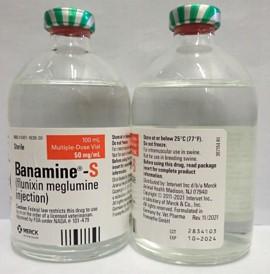
- BANAMINE-S 100mL, UIN 065477, NDC 0061-1838-30, Batch 2834103, exp Oct. 2024
o Distribution dates: March 20, 2023, to May 16, 2023
A photo of the bottle of each batch number (lot number) that is part of the recall can be found at the end of this press release. The lot number (LOT) and expiry date (EXP) is located at the bottom right part of the bottle label.
The administration of an injectable product that contains particulate matter may result in local irritation, swelling or infection in response to the foreign material. After intravenous administration in large animals, such as cattle or horses, particulate matter could travel to the lungs which could result in local tissue damage. To date, no adverse event reports have been received for the recalled batches.
Flunixin meglumine is a potent, non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It is approved in the U.S. only for intravenous use in beef and dairy cattle, for intravenous and intramuscular use in horses and for intramuscular use in swine.
Instructions and questions
Customers who have received BANAMINE and BANAMINE®-S from the batches being recalled should stop using the products and refer to their recall letter for product return instructions. Merck Animal Health is working with our distributor partners to ensure that unused product is no longer in distribution or with customers. We are notifying our distributors and customers directly and are arranging for the return of all recalled product.
Consumers with questions regarding this recall should call 1-800-521-5767 (Monday through Friday 8 a.m. – 5 p.m. CDT).
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA at 1-888-FDA-VETS or online at http://www.FDA.gov/reportanimalae.
This recall is being made with the knowledge of the Food and Drug Administration.
About Merck Animal Health
The health and well-being of animals is the foremost priority at Merck Animal Health.
The company is present in more than 50 countries, while its products are available in some 150 markets. For more information, visit www.merck-animal-health.com.
Product Photos
Forward-Looking Statement of Merck & Co., Inc., Rahway, N.J., USA
This news release of Merck & Co., Inc., Rahway, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of the global outbreak of novel coronavirus disease (COVID-19); the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s Annual Report on Form 10-K for the year ended December 31, 2022 and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov).